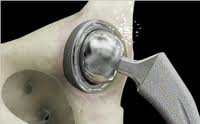
Public Health Nightmare - Implant "innovation"
published August 10, 2011
"no one's interest is served by putting defective medical devices onto the market where they cause harm to patients, waste health dollars, and may kill jobs when they (device products) are withdrawn."
Joint replacements are the #1 expenditure of Medicare. The process of approving these medical devices is flawed according to the Institute of Medicine. It is time for patients' voices to be heard as stakeholders and for public support for increased medical device industry accountability and heightened protections for patients. Post-market registry. Product warranty. Patient/consumer stakeholder equity. Rescind industry pre-emptions/entitlements. All clinical trials must report all data.
Please share what you have learned!
Twitter: @JjrkCh
Subscribe to:
Post Comments (Atom)

No comments:
Post a Comment